Development of HPV16 mouse and dog models for more accurate prediction of human vaccine efficacy
As observed with the COVID-19 pandemic, the lack of appropriate preclinical animal models is a major obstacle to the development of specific therapies. Existing transgenic mouse lines are not sufficient for preclinical research into therapeutic HPV vaccines.
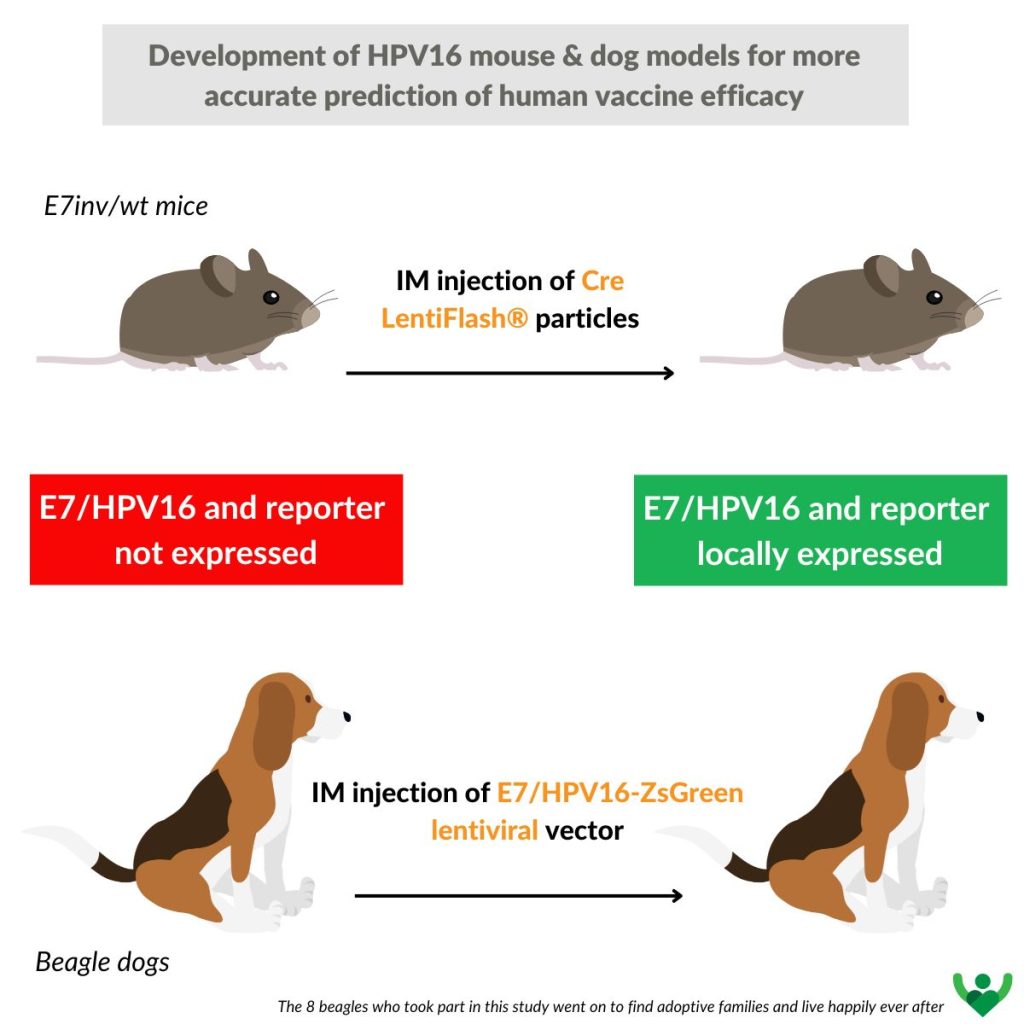
The main aim of this study was to develop new animal models for evaluating the immune response and efficacy of vaccine candidates against HPV infections. Two complementary approaches were tested to obtain animals expressing HPV E7 antigen using two different animal models, the mouse and the dog.
The study reports the lack of relevant animal models for therapeutic vaccination, in this case for infections with the HPV virus responsible for several types of cancer, including cervical cancer.
For this study, a C216 vaccine candidate, similar to ProCervix, was used to validate two new complementary preclinical models, in mice and dogs* (whose immune systems are very similar to those of humans, and which represent the diversity of the human MHC, unlike mice). Since HPV16 is the most common cause of cervical cancer (50%), the study focused on this particular valency.
The mouse model is an E7/HPV16 transgenic mouse in which expression of the HPV16 E7 antigen occurs only after Cre-lox recombination. LentiFlash® non-integrating particles (now FlashRNA®) are used here to locally deliver Cre recombinase RNA and enable expression of the E7 antigen and a fluorescent reporter, for local in vivo analysis. A conventional lentivector was used in dogs to locally express HPV16 in muscle at the time of vaccine candidate testing.
These approaches make it possible to measure the efficacy of the candidate vaccine by eliminating cells expressing the HPV16 E7 antigen, which is linked to the fluorescent reporter.
Vaccination with C216 did not induce a sufficiently strong immune response to eliminate infected cells, which is in line with the results of the ProCervix Phase II clinical trial and demonstrates the need for more relevant pre-clinical animal models.
For further information : C216 is a new trivalent E7/HPV therapeutic vaccine candidate very similar to ProCervix (GTL001). Both candidates are based on the CyaA platform developed by Institut Pasteur. CyaA is a recombinant detoxified adenylate cyclase toxin (CyaA) originally isolated from Bordetella pertussis hemolysin.
In more detail, C216 carries the non-oncogenic fused antigens E7/HPV-16, E7/HPV-18, E7/HPV-45, while ProCervix carries non-oncogenic E7 and the HPV-16 and E7/HPV-18 antigens.
CyaA targets antigen-presenting cells to initiate a specific CD8 T cell response. It can deliver multiple epitopes without loss of immunogenicity. This system also leads to antigen presentation by major histocompatibility complex (MHC) class II.
To see the full article : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10258489/
Authors : Totain E, Lindner L, Martin N, Misseri Y, Iché A, Birling MC, Sorg T, Herault Y, Bousquet-Melou A, Bouillé P, Duthoit C, Pavlovic G, Boullier S. Development of HPV16 mouse and dog models for more accurate prediction of human vaccine efficacy. Lab Anim Res. 2023 Jun 12;39(1):14. doi: 10.1186/s42826-023-00166-3. PMID: 37308929; PMCID: PMC10258489.
*No dogs were mistreated. All dogs are part of a specific program and have been adopted into loving families.