Regenerative medicine
Lymphedema treatment using gene therapy
Regenerative medicine is transforming healthcare by enabling the repair, replacement, or regeneration of organs, tissues, cells, genes, and metabolic processes in patients. By leveraging advances in gene and cell therapies, this field aims to expand the range of conditions that can be treated, including numerous previously deemed incurable. From stem cell therapies to tissue engineering and the use of gene therapy in various combinations, regenerative medicine is paving the way for personalized healthcare.
Flash BioSolutions is a pioneer in this regard, offering state-of-the-art gene therapy solutions. The TheraLymph project is a groundbreaking example, addressing lymphedema and providing new hope for patients affected by this disabling condition.
Medical Context
Between 10% and 15% of women develop secondary lymphedema after breast cancer surgery. This common disorder of the lymphatic vascular system affects over 120 million people worldwide, causing significant morbidity.
Characterized by impaired lymphatic return and swelling of the extremities, lymphedema results in the accumulation of undrained fluid/lymph, leading to fibrosis and adipose tissue deposition in the affected arm or leg. While it can be inherited, it more commonly occurs following cancer surgery and lymph node removal. Unfortunately, there is currently no curative treatment for lymphedema.
Objectives & PROGRAM challenges
TheraLymph program: A dual RNA Therapy to restore lymphatic flow in patients with lymphedema
The main objective of the TheraLymph project is to develop a Lymphedema treatment using FlashRNA® multi-RNA delivery technology in a non-integrative gene therapy approach. The project consortium’s translational research program brings together scientists and physicians from 5 European countries and is focused on patients who developed Lymphedema after breast cancer surgery.
The project goal is to validate the best mRNAs combination for gene therapy and to finalize phase I/II clinical trial at the affiliated hospital.
Timeline & Funding
The Theralymph project was launched in 2020 for the duration of 5 years and has received funding from the EU Horizon 2020 research and innovation program under Grant Agreement No 874708.



PROGRAM PHASES
- In vitro and in vivo validation of therapeutic targets identified by the different partners, such as VEGFC associated to another factor to restore the lymphatic collecting network.
- In vivo determination of the efficacy of FlashRNA® particles as an RNA delivery solution compared to an integrative lentivector, in lymphedema mouse models.
- Regulatory pre-clinical testings on rabbit to assess biodistribution and toxicity.
- Phase I/II clinical testing in patients using FlashRNA® multi-RNA delivery technology.
main Results
Promising preclinical outcomes
Our preclinical results showcase the remarkable effectiveness of FlashRNA® as a single delivery system for two distinct therapeutic RNAs-Apelin and Vascular Endothelial Growth Factor C. A mouse model of secondary lymphedema was used to evaluate and validate the effectiveness of this dual RNA therapy.
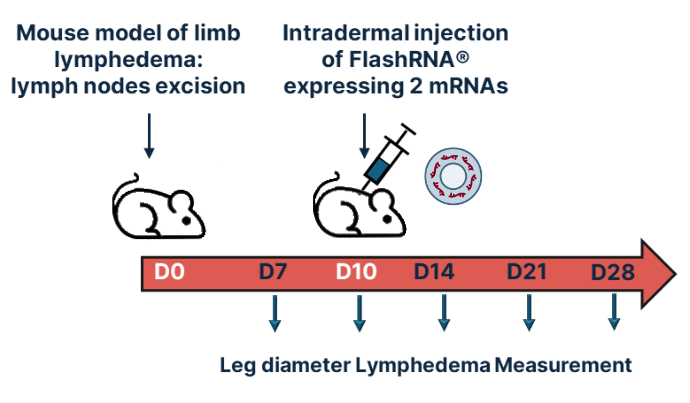
Description of the in vivo model used to evaluate the dual mRNA Therapy (Apelin & VEGF-C)
D0: surgical model generation. A secondary lymphedema is induced in mice through mastectomy of the second mammary gland, along with brachial and axillary lymph nodes removal
D10: treatment. FlashRNA® is injected intradermally into the mouse limb. The assessment of lymphedema is conducted through the measurement of limb diameter and the visualization of dermal backflow through angiography.
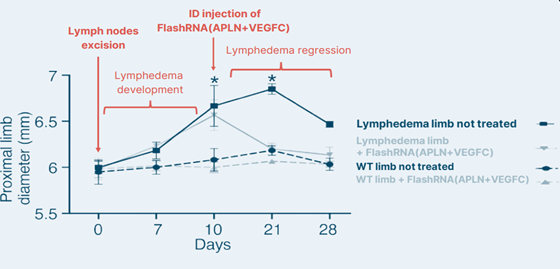
Efficacy in reducing Lymphedema symptoms
The administration of FlashRNA® carrying APLN and VEGF-C mRNAs yielded remarkable results in vivo:
- Swelling reduction, starting within 1 day of treatment.
- Complete regression of lymphedema achieved 11 days post-treatment, whereas the pathology usually regresses naturally in 21 to 28 days.
Restoration of lymphatic vessels & suppression of dermal backflow typically observed after lymph nodes resection (White arrows in left panel of figure below “Control group – LD leg”).
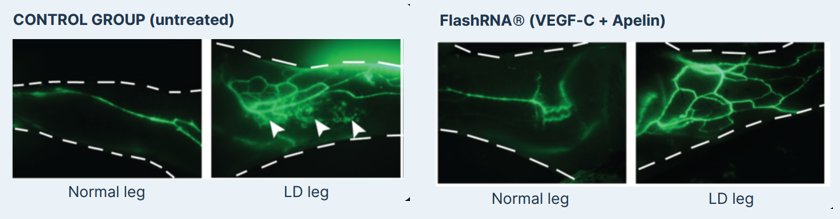
These findings highlight the potential of using FlashRNA® to deliver both Apelin and VEGF-C RNAs into cells to help them regenerate and restore lymphatic function. This is an important step towards new treatments for regenerative medicine.
Perspectives
As the Theralymph project moves forward, it is pioneering a significant transformation in the treatment of lymphedema, including cases of primary lymphedema in the near future. We envision a future where millions of people worldwide can benefit from innovative solutions, improving their quality of life and installing new hope.
The potential of this innovative RNA transfer technology extends beyond regenerative medicine. It has the capacity to drive significant advancements in gene and cell therapy. By enabling precise delivery of therapeutic RNA, FlashRNA® can facilitate the correction of genetic defects and enhance immune cell therapies or vaccination approaches. As research progresses, RNA transfer technology has the potential to revolutionize how we approach a wide range of diseases, transforming treatment paradigms in gene and cell therapy.
Would you like to discuss the subject with us ?

For more information, please visit the Theralymph project website and donwload the Poster.
Consortium members
University of Helsinki
This project will be carried out in the Biomedicum Helsinki Research Center, which is a unique environment, one of few in Europe where all fields of biomedical research are located in the Academic Medical Center Helsinki (AMCH). The Translational Cancer Biology group, led by Dr. Alitalo, investigates the induction of lymphatic vessel growth and its benefits in lymphedema, using viral vector transduction, as well as the organ-specific heterogeneity and functions of lymphatic vessels using a combination of state-of-the-art molecular, cellular and genetic in vivo methods.
The group also investigates the role of meningeal lymphatic vasculature in neuroinflammation and neurodegenerative diseases. The team further characterizes responses of lymphatic vessels to cancer therapies, with a focus on clinically relevant immunotherapy approaches.
University of Lausanne
Vascular and Tumor Biology Laboratory is part of the Department of Oncology of UNIL and is an adjunct member of Lausanne branch of Ludwig Institute for Cancer Research Lausanne, that is specializing in tumor immunology and immune therapy. Their main research interests are in the molecular mechanisms of lymphatic and blood vessel growth and remodeling, and their role in normal organ function and diseases, such as inflammation and cancer.
University of Uppsala
The Vascular Development Laboratory headed by Dr. Mäkinen develops and utilizes genetic mouse models in combination with molecular and cell biological approaches to study mechanisms that regulate the morphogenesis and functional specialization of the vasculature. The lab also investigates how regulators of developmental (lymph)angiogenesis impact on genetic human diseases such as lymphoedema and vascular malformations.
University of Liège
The Laboratory of Biology of Tumor and Development (LBTD) belongs to the GIGA-Cancer in the GIGA Research Center (ULg), a major multidisciplinary center of research in life sciences composed of >600 researchers. In this center, team members have access to fully equipped and staffed core facilities and platforms. One of the main topics of research include cancer and tumor microenvironment, with a particular interest on angiogenesis and lymphangiogenesis in the context of breast cancer.
De Duve Institute
The Laboratory of Human Molecular Genetics, headed by Prof Miikka Vikkula, MD, PhD, focuses on characterization of the underlying pathophysiology of vascular anomalies, such as lymphedema, as well as cleft lip and palate, and selected cancers. The long-term goal is to develop molecular precision therapies for these disorders. The lab is specialized in evaluating the contribution of genetic variation to human disease. This research is based on blood and tissue samples collected from patients in collaboration with clinicians and multidisciplinary centers worldwide.
The lab analyses patients’ genomes using highthroughput (NGS) sequencing, including targeted panels, WES (whole exome sequencing), WGS (whole genome sequencing) and RNAseq. With its full-time bioinformatician, the group has developed an in-house software called Highlander implementing specialized bioinformatic tools for NGS analyses.
Charles University
The Laboratory of Pathophysiology of Adipose Tissue presented a number of important contributions in studies focused at changes of metabolic, endocrine and immune characteristics of AT during lifestyle – diet and physical activity- interventions in obese population. The main aim of the present activity is to elucidate a role of lipogenic and adipogenic capacity and inflammatory state of AT in ethiopathogenesis of obesity, type 2 diabetes, lymphedema and in pathogenesis of metabolic disturbances associated with aging. Laboratory is focused on clinical and translational research combining clinical studies with patients and in vitro laboratory techniques to analyze processes that underlie, or mediate disturbances related to dysfunctional adipose tissue.
Inserm
The PI laboratory is located in the Institute of Cardiovascular and Metabolic Diseases (I2MC) created in 2011 by Inserm. The research activity focuses on metabolic, cardiovascular and renal diseases. The main feature of I2MC is the gathering of basic scientists together with clinicians working on metabolic risk factors (obesity, diabetes and dyslipidemia) and their cardiovascular complications (thrombosis, atherosclerosis, cardiac and renal failure).
The laboratory headed by Dr Garmy-Susini studies molecular regulations of (lymph)angiogenic factors in vascular pathologies. It developed research axes in the field of gene expression control in response to stress and in pathophysiology of the lymphatic system, connected to therapeutic axes of gene therapy of ischemic heart disease and lymphedema. The main objectives of the lab are to 1/identify the pathophysiology of the lymphatic system in lymphedema, 2/characterize regulation of lymphangiogenesis-related gene expression in stress conditions (hypoxia, ER stress…), and 3/develop innovative therapies to restore lymphatic flow to improve healing in ischemic heart and in lymphedema.
CNRS
The project is held by the Institute for Research on Cancer and aging of Nice (IRCAN). The team of Gilles Pagès focuses its research on the regulation of angiogenic factors and their role as markers of sensitivity or resistance to different cancers treatments. The first major paper of the team described the efficacy of Gleevec in chronic myeloid leukemia; (Legros, L et al Blood, 2004). More recently the team focused on renal cell carcinoma and published pivotal papers on the mechanisms of resistance to anti-angiogenic drugs.