ruo viral vector
services
Over 20 years of expertise in viral vector production, providing gene delivery systems for your research success.
Ready to take your research projects to new heights? Choose excellence with our standard or customized gene delivery solutions. With our well-established viral vector production process and experienced experts by your side every step of the way, we ensure optimized viral transduction of your cells.

HIGHLIGHTS OF OUR SERVICES

Track record in vector production

11 000+ batches manufactured
3 weeks
turnaround time
One
stop shop
More than 2000 global clients are trusting us
LentiCare® grades
FlashRNA® grades
lentiVIRAL VECTOR GRADES
Discover our range of integrative viral vector services tailored to meet your needs: LentiCare® technology.
To address your specific needs, we have tailored our production and purification process to offer you 3 types of LentiCare® batches, each designed to perfectly suit the applications of your projects:
- Ready range
- Start range
- Premium range
flashrna® particles GRADES
Discover our range of RNA delivery solutions tailored to meet your needs: FlashRNA® technology.
To cater to your specific needs, we have tailored our production and purification process to provide you with 2 types of FlashRNA® batches, each ideally suited to the requirements of your projects:
- Start range
- Premium range
Recombinant viral vector services
Recombinant lentiviral vectors are powerful tools for delivering genes into mammalian cells. We have developed an optimized lentiviral system with the highest safety features. Our vector packaging services involve co-transfection of the transfer plasmid containing the gene of interest (GOI), the envelope plasmid (classically expressing VSV-G), and the packaging plasmid(s) into producer cells.
Once produced, viral vectors are highly purified and concentrated enabling safe use for in vitro and in vivo research applications.
We understand the crucial importance of reliable and safe viral vector production. That’s why we have optimized our protocols for manufacturing high-quality recombinant viral vectors to enhance titer, purity, and efficacy.
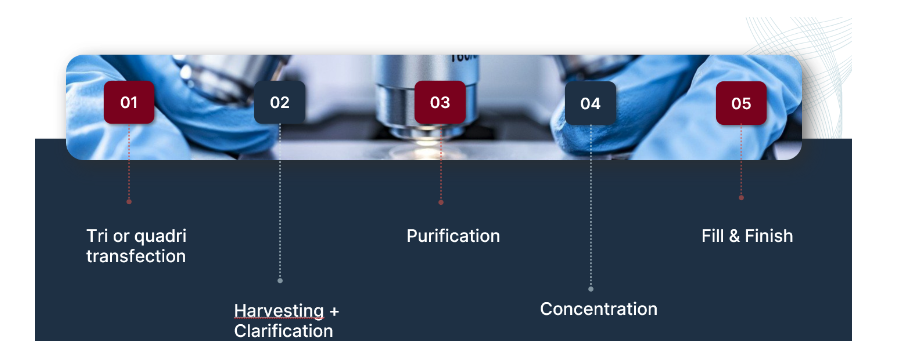
Robust and well-established bioproduction process
QC and robust titration methods
We’ve developed a purification process for the production of high-purity, high-titer viral vectors that comply with cGMP standards, ensuring that residual protein and DNA impurity levels meet the drug product specifications. This ensures optimal preservation of transduced cell phenotypic profiles and cellular responses.
At Flash BioSolutions, each construct undergoes rigorous quality control procedures, reflecting our commitment to excellence. We meticulously measure the functional titers of viral vectors, provided in Integrated Genomes per milliliter (IG/ml), by using qPCR to specifically target a unique sequence in the vector genome, ensuring unparalleled accuracy. This precise methodology provides a clear view of efficient particles, thereby delivering the real number of integrative particles crucial for the success of your research projects.
For more in-depth quality control needs in your research projects, we also offer additional analyses and titration methods such as the p24 ELISA assay, giving titers in Physical Particles per milliliter (PP/mL) and the Transgene Expression Assay (TU/mL), see table below.
| Standard QC Methods | Description | Viral titers specifications for a Premium batch |
|---|---|---|
| Titration by ELISA (PP/mL) | Physical particles : Quantification of viral structure element (p24 viral capsid protein) directly in the viral supernatant by ELISA, taking into account functionnal and non-functionnal vector particles. | > 1E11 PP/mL |
| Titration by qPCR (IG/mL) | Functional viral titer : Quantification of the number of integrated genomes by qPCR (true count of functional particles) and assessment of their integration capacity. | > 1E9 IG/mL |
| Transgene Expression Assay (TU/mL) | Specified according to your needs : Accurate assessment of the dose effect linked to the expression of transgenres of interest, by direct detection of fluorescent reporters or by specific immunostaining using flow cytrometry. | > 1E9 TU/mL |
Standard
viral vectors
Ready-to-use viral vectors guaranteeing up to 100% transduction efficiency on primary cells, stem cells and cell lines.
Custom
viral vectors
Tailor-made viral vectors are perfectly fitted to your requirements
FAQ
How are Flash BioSolutions viral vectors different from other viral vectors?
Flash BioSolutions has optimized a purification and concentration platform specifically for viral vector production. Our production processes are customized based on the target cell type, ensuring high titer and exceptionally pure viral particles which allow to fully maintain target cell viability, phenotype, and proliferation capacities.
What is the difference between Transduction Units (TU)/ Integrated Genome (IG) and Physical Particles (PP)?
During the production of viral vector, both efficient and non-efficient particles are produced. Efficient and complete particles are quantified by qPCR as integrated genome (IG) and by cytometry as transduction unit (TU). Physical Particles (PP) are quantified by p24 ELISA and represent all particles, i.e., not only efficient particles but also damaged/empty particles and free p24 protein (see figure below). Consequently, the PP/TU or PP/IG ratio is a quality control criterion for the vector product, as it is better to have a minimum of damaged/empty PP in the viral supernatants to retain only the efficient particles. Reliable and discriminating titration methods are therefore needed for the different types of particles.
A titer in TU/ml or IG/ml is required for an accurate calculation of effective vectors applied to the cells. The IG/mL or TU/mL titer provided by Flash BioSolutions is a precise titer of effective viral particles. It represents the titer determination of integrative particles only. Calculating the volume of vectors to be used using such titration is then more accurate, and experimental results can be extrapolated and compared from one experiment to another.
How is the functional titer in IG/mL of the viral vectors determined?
The titer information provided by Flash BioSolutions is a functional titer determined by qPCR measurement of only the viral particles that are able to transduce cells (Integrated Genome/mL), giving a measure of the vector’s potency. Specifically, IG corresponds to the number of integrated genomes generated by transducing HCT116 cells with several serial dilutions of viral supernatant. Data are reviewed and validated as part of quality control to ensure that the viral vectors meet the required specifications.
How is the functional titer in TU/ml of the viral vectors determined?
The titer information provided by Flash BioSolutions can be a functional titer determined by flow cytometry measurement of only the viral particles that are able to transduce HCT116 titration cells (TU transducing Unit/mL). Specifically, the titer deduced from FACS experiments corresponds to the level of expression of the protein of interest and, as such, can be considered as a functional titer. Data are reviewed and validated as part of quality control to ensure that the viral vectors meet the required specifications.
What are the differences in purity levels and quality control measures based on the viral vector range selected?
Our viral vector platforms are tailored to meet the specific needs of different applications, with each platform offering distinct levels of purity and quality control measures to ensure optimal performance. We offer a comprehensive range of options designed to meet different vector production needs. The entry-level VectaStart offers reliable performance for a wide range of applications. It is optimized for larger, high-throughput needs where extreme purity is not as critical. It is suitable for initial research, high-volume screening? and applications where cost-effectiveness is a priority over the highest possible purity levels. VectaPremium is the advanced offering in the Vecta series, engineered to deliver superior purity and quality. It undergoes enhanced purification processes to guarantee higher levels of vector purity, including advanced filtration techniques that minimize contaminants and residues, ideal for applications requiring the highest purity standards, where even minimal contaminants could impact outcomes or safety.
In addition, the production report of the transfer plasmid batch used for viral vector production is provided, as well as the plasmid cloning report, when the transfer plasmid has been constructed on demand.
How many viral particles should I add to my cells?
Because the titer of each batch of viral particles varies, the volume required for transduction will also vary. The titer can be found on the Certificate of Analysis (CoA) provided with each viral vector batch. Use the formula detailed in the Customer Recommendations document to calculate the volume of integrative viral vector for transduction. Please refer to the other customer recommendations manual for additional information.
Viral vectors volume required (µl) = Number of cells to transduce / Viral vectors Titer (IG/ml) x Multiplicity of Infection (M.O.I) x 1000
What are the safety concerns about the use of viral and retroviral vectors?
These products are designated as biosafety level 2 biological agents. Follow the biosafety procedures for use of retroviral-derived vectors in accordance with all applicable regulations. All Flash BioSolutions manufactured viral vectors are produced from separate transfer, envelope, and packaging components onto different plasmids, which results in the generation of replication-incompetent vectors. Furthermore, all transfer plasmids include two additional safety features, with the use of a chimeric tat-independent 5’LTR and the self-inactivating (SIN) modification of the 3’ LTR. As a result, while the viral vectors maintain the ability to transduce a wide range of cell types, they are not capable of producing replicative particles from transduced cells, as assessed by RCL/RCR testing. Please refer to the MSDS documents for more information.
How should the viral vectors be stored?
The viral vectors must be stored at -80°C immediately upon receipt.
How should viral vectors be thawed?
Vectors must be taken out of the -80°C freezer at the last moment. On removal from the -80°C freezer, viral vectors must be placed on ice to begin the thawing protocol. Once thawed, the vectors must be rapidly used for transduction. It is also essential to avoid thermal shock when handling viral vectors. If vectors are to be diluted in cell culture medium, use medium at room temperature once the vector has thawed, to avoid thermal shock and ensure particle efficiency.
Thaw the particles according to the volume of your vial, as described below.
For a volume of viral vectors higher than 1mL: Just before transduction, take the tubes of viral vectors out of the -80°C freezer and thaw them at room temperature or in a 37°C water bath. Before the last ice-cube disappears, place the tube at room temperature to complete thawing and start transduction.
For a volume of viral vectors between 1mL and 100µL: Just before transduction, take the tubes of viral vectors out of the -80°C freezer and thaw them at room temperature or in a 37°C water bath. Before the last ice-cube disappears, place the tube at room temperature to complete thawing and start transduction.
For a volume of viral vectors lower than 100µL: Just before transduction, take the tubes of viral vectors out of the -80°C freezer and thaw them on ice at 4°C. 5 minutes before transduction take the tubes out of the ice and allow them to warm to room temperature.
Can the viral vectors be thawed and refrozen?
Viral vectors are sensitive to freeze-thaw cycles. Flash BioSolutions therefore recommends avoiding freeze-thaw cycles for your viral vectors, which is why they are packaged in several aliquots. If absolutely necessary, we recommend aliquoting viral vectors on ice in working volumes for your experiments, when you perform your first transduction experiment. An increasing reduction in titer for every freeze-thaw cycle should be expected.
To avoid this aliquoting step, you can request a specific aliquoting for your project when you place your order.
Can the viral particles be used more than once?
We do not recommend using the same aliquot of viral vectors more than once. In addition, the remaining volume of any thawing viral vectors should be discarded after the transduction experiment.
What type of medium should be used during transduction of my cells?
The optimal media will vary based on the cell type. The best medium to use is usually the medium used for your cells’ culture. No need for any specific medium related to transduction using viral vectors.
Can the CRISPR-Cas9 system be used for screening?
The CRISPR-Cas9 system is widely used for functional genomic screening, with single-guide RNA (sgRNA) libraries, targeting thousands of genes and allowing screening for gene activation or inhibition. These libraries are commonly packaged into viral vectors. When multiple transductions are required, how long should I wait before the second transduction? If necessary, we recommend waiting a minimum of 48 hours before performing a second transduction.
Is it possible to perform specific cell targeting with your vectors? We developed a range of viral vectors allowing specific gene expression in particular tissues, cell types or cell states. With reporter expression under the control of specific promoters, these vectors enable to label specific tissues (liver, pancreas,…), subcell types in a tissue (neurons, astrocytes, germline cells,…) or cells in a specific state (proliferative, senescent, …).
Do you produce viral CRISPR/Cas9 libraries?
We can produce pooled viral sgRNA libraries, starting from a provided transfer plasmid library, expressing the different sgRNAs (as well as Cas9 coding sequence, if required). The vector concentration grade and final volume will have to be adapted to the library complexity, to ensure a sufficient number of integrations per sgRNA in your screening experiment.
Do you provide viral vectors capable of engineering difficult-to-transduce cells ?
We achieve excellent transduction rates for all cell types, including those difficult to transduce, such as primary cells and stem cells. For difficult-to-transduce cells or sensitive cells, we recommend using the Premium grade to achieve the highest transduction efficiency.
CONTACT US
Fill in the form below and our team will contact you shortly to explore how we can help you turn your ideas into success.
+33(0) 5 61 28 70 75









